Delivering a new era in low volume irrigation
Launched in April 2021 Qufora IrriSedo MiniGo and MiniGo Flex aimed to reimagine low volume irrigation by making it fit easily into patient’s everyday lives. This page is dedicated post market surveillance, in which we looked for detailed feedback from a number of healthcare professionals using Qufora IrriSedo MiniGo and MiniGo Flex.
In 2024, the MiniGo and MiniGo Flex cones were redesigned and a MiniGo Flex cone small designed for paediatric patients was added to the range. In initial patient evaluations 82% of patients agreed or strongly agreed that they would carry on using MiniGo if it was available. 90% of patients who trialled the MiniGo Flex small agreed to the same statement.
It’s fundamental that our approach to designing effective low volume irrigation solutions supports those healthcare professionals prescribing and teaching the system. But importantly is centred around those that use it.
Good intentions delivered
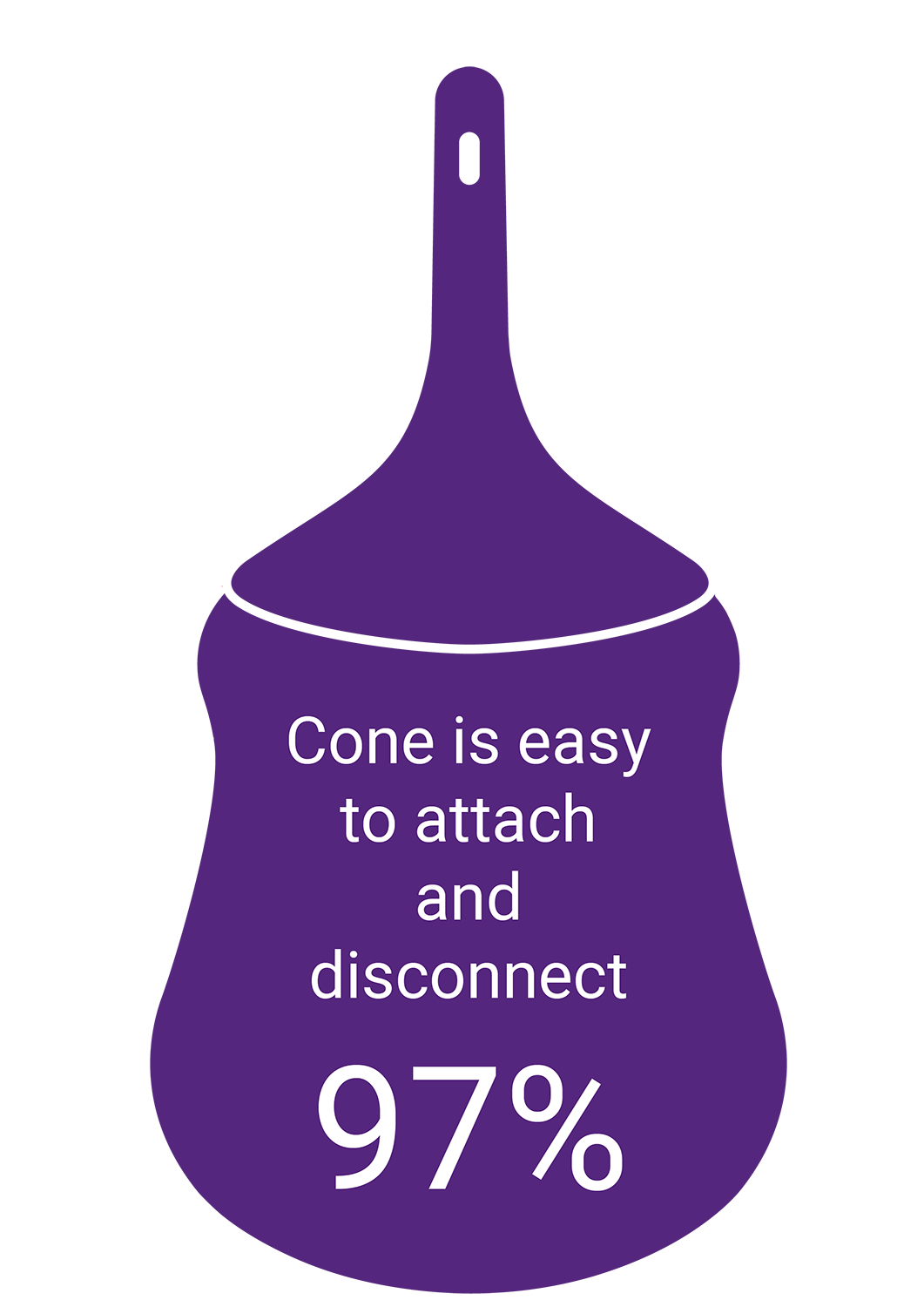
We set out to create an effective low volume irrigation system with features designed to make it easier to use. It was also essential to make it as discreet as possible. Here’s what healthcare professionals said.


Good intentions delivered
We set out to create an effective low volume irrigation system with features designed to make it easier to use. It was also important to make it as discreet as possible.
Features that make a difference









An innovative cone designed for comfort

- Rounded tip for comfortable insertion
- Oval design to fit the buttock shape
92%
agree the cone lubricant was sufficient for insertion
92%
We were the first to introduce a low volume irrigation system in 2013
Features that make a difference







93% said the box is discreet and 87% said the cone lubricant was sufficient for insertion
The system has been life changing and has really helped me.
What patients are saying


Evaluation results and background
Post market surveillance (PMS) of a product with a healthcare professional (HCP) is central to appraising the components of a product. In addition, it is fundamental to quantify the ease with which an HCP can deliver the educational content and technique of a product to a patient. This in turn ensures that the HCP and Qufora are working and succeeding together by providing high quality benchmarked information. Furthermore, this assists to reinforce claims made at the product launch.
The purpose of this post market evaluation of the Qufora IrriSedo MiniGo/ GoFlex with healthcare professionals (HCP) is to ensure the product meets with the fundamentals of the ‘Clinical Evaluation Report’ and follows the guidelines of Articles 83, 84 and 85 in MDR 2017/745 (Appendix 3). Additionally, PMS can also assist in identifying areas for development and ultimately provide an important basis for product quality improvement. This gives an insight into the needs of the patient and the HCP, which is aligned to the Qufora mission, vision, and values.
Post market surveillance (PMS) of both Qufora IrriSedo MiniGo and MiniGo Flex was undertaken with patients who use them for irrigation. The survey focussed on evaluating the packaging, the performance of the soft pump and cone /cone flex and the flip lid. The overall performance in terms of ease of use (assembling and learning how to use) was also appraised.

